Contract Development & Manufacturing (CDMO) is a pilar service we have been offering for more than 20 years alongside with experience, agility, innovation, and regulatory expertise in inorganic and organic salts.
We harness this experience and a state-of-the-art facility that includes diverse teams of highly experienced scientists and regulatory technicians to collaborate with you on a tailored project.

We are committed to executing projects seamlessly through maintaining optimized & cost-effective manufacturing processes that do not compromise transparency nor quality.
Benefits of our CDMO Service
CDMO Service Outline
Our service starts with you selecting us as your reliable CDMO and taking advantage of our know-how.
The service outline includes 10 steps that are modified based on GMP or non-GMP (ISO) requirements of the project.
Our priority is to align with your expectations in terms of specifications, regulatory support, timeline, and cost.
Portfolio Scope
Our product portfolio of mineral salts includes the following elements:
 Aluminium is the most widely-used non-ferrous metal
Aluminium is the most widely-used non-ferrous metal Precious metal, been used in the manufacture of coins,ornaments and jewelery.
Precious metal, been used in the manufacture of coins,ornaments and jewelery. Barium is used in drilling fluids for oil and gas wells. It is also used in paint and in glassmaking.
Barium is used in drilling fluids for oil and gas wells. It is also used in paint and in glassmaking. Cubic solid, the most abundant metallic element in the human body and the fifth most abundant element in Earth's crust
Cubic solid, the most abundant metallic element in the human body and the fifth most abundant element in Earth's crust Heavy metal used especially for heat-resistant and magnetic alloys
Heavy metal used especially for heat-resistant and magnetic alloys Rubies get their red colour from chromium, and glass treated with chromium has an emerald green colour.
Rubies get their red colour from chromium, and glass treated with chromium has an emerald green colour. Historically, copper was the first metal to be worked by people.
Historically, copper was the first metal to be worked by people. Iron is an enigma – it rusts easily, yet it is the most important of all metals. 90% of all metal that is refined today is iron.
Iron is an enigma – it rusts easily, yet it is the most important of all metals. 90% of all metal that is refined today is iron.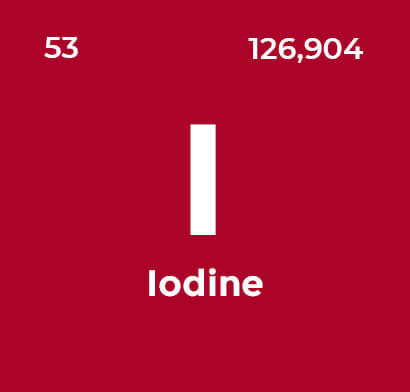 Iodine and its compounds are primarily used in nutrition
Iodine and its compounds are primarily used in nutrition Potassium levels influence multiple physiological processes and is the eighth or ninth most common element by mass (0.2%) in the human body
Potassium levels influence multiple physiological processes and is the eighth or ninth most common element by mass (0.2%) in the human body Lithium was discovered from a mineral, while other common alkali metals were discovered from plant material
Lithium was discovered from a mineral, while other common alkali metals were discovered from plant material Magnesium is the third-most-commonly-used structural metal, following iron and aluminium. It is very abundant in cells.
Magnesium is the third-most-commonly-used structural metal, following iron and aluminium. It is very abundant in cells. Manganese is an important element for human health, essential for development, metabolism, and the antioxidant system
Manganese is an important element for human health, essential for development, metabolism, and the antioxidant system About 86% of molybdenum produced is used in metallurgy, with the rest used in chemical applications.
About 86% of molybdenum produced is used in metallurgy, with the rest used in chemical applications. Sodium is a soft metal that tarnishes within seconds of being exposed to the air. It also reacts vigorously with water.
Sodium is a soft metal that tarnishes within seconds of being exposed to the air. It also reacts vigorously with water. A silvery metal that resists corrosion even at high temperatures
A silvery metal that resists corrosion even at high temperatures Lead has many uses in the construction industry;
Lead has many uses in the construction industry; The biggest use of selenium is as an additive to glass
The biggest use of selenium is as an additive to glass Strontium was mostly used in the production of sugar
Strontium was mostly used in the production of sugar Most zinc is used to galvanise other metals.
Most zinc is used to galvanise other metals.